PRODUCTS
Product Name: |
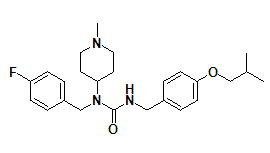
706779-91-1 | Pimavanserin |
CAS NO: |
706779-91-1 |
Molecular formula: |
C25H34FN3O2 |
Molecular weight: |
427.55 |
Structural formula: |
 |
Product Description: |
Pimavanserin/Pimavanserin (Nuplazid) was granted breakthrough therapy certification by the U.S. Food and Drug Administration (FDA) on September 3, 2014. The breakthrough therapy certification was created by the FDA to accelerate the development and review of new drugs for the treatment of serious or life-threatening diseases. Acadia Pharmaceuticals will submit a new drug application for Pimavanserin/Pimavanserin to the FDA before the end of 2014. Dr. Cummings of the Lou-Ruvo Brain Health Center in Cleveland and others conducted a phase III clinical study of pimavanserin for the psychiatric symptoms of Parkinson's disease, and further its efficacy and safety under the premise of reducing the effect of placebo The evaluation was carried out, and the relevant results were published in The Lancet on February 8, 2014 (The Lancet, Volume 383, Issue 9916, p533-540). Studies have found that Pimavanserin can improve the mental symptoms of patients with Parkinson's disease. The study included 199 patients from August 11, 2010 to August 29, 2012, of which 95 were treated with pimavanserin and 90 were treated with placebo. The SAPS-PD score of patients in the pimavanserin drug group decreased by 5.79 points, while the placebo group only decreased by 2.73 points. |


